Tuesday, May 18, 2010
Tuesday, May 11, 2010
May 10
- Carbon compounds can form rings
- Follow the same naming rules and add cyclo- in front of the parent chain
- When numbering, you can count clockwise or counterclockwise, but where you start (#1) should be on one of the side chains because you are using the lowest numbering system
Examples of Cyclo Alkanes:
- When a cyclic 6 carbon chain forms, it can create a resonance structure called Benzene

Monday, May 10, 2010
Organic chem
- Compounds with double bonds end in -ene
- Put a # in front of the parent chain that indicates where the double bond is
- More thn one double bond changes the parent chain slightly
- Double bond always has priority (when choosing the direction of numbering)
- Any rime more than one double bond occurs, you add adi, atri, atetra...
- The longest chain has to include the double bond

Alkynes
- For compounds with triple bonds use -yne ending
- Follow all the same alkene rules
- The longest chain has to include the triple bond

ORGANIC CHEMISTRY
- There are more carbon compounds than all ionic compounds combined
- The study of carbon compounds is called organic chemistry
- Carbon can have multiple bonds and form many different shapes
- Hydrocarbons have three types of formulas:
1) Molecular formulas
C6H14
2) Condensed Structural Formula
CH3-CH2-CH2-CH2-CH2-CH3
3) Structural Formula

Nomenclature of Hydrocarbons
- One molecular formula can have a number of different structures
- Isomers are compounds that can be drawn in more than one way
Naming Alkanes
1) Name the longest chain by using the correct suffix and adding "ane"
2) Locate any branches by number carbon atoms (use the lowest possible number system)
3) Name branches by using appropriate suffix and -yl ending (Alkyl branches)
4) If there are more than one of the same alkyl group, number each one and add the multiplier number in front of the branch name

Tuesday, April 20, 2010
April 20, 2010
Polar substances have an unequal charge distribution (asymmetrical)
H2O = polar:
LIKE DISSOLVES LIKE!
Polar solutes dissolve in polar substances and non-polar solutes dissolved in non-polar substances
___________________________________________________________
INTERMOLECULAR BONDS
- bonds between molecules
- 3 types
1.)
- Results from temporary electron dipoles
- Weakest intermolecular force
- Increases as the $ e- increases
- Occurs in any compound that has e- (ie: everything)
2.) Dipole Dipole
- Results from a permanent dipole in molecules
Polar molecules experience this force
Polarity depends how much elements want e- (electronegativity)
- Electronegativity increases to the right and up
- The strength of a dipole- dipole bond depends on the difference in electronegativity between the two atoms
- Only polar molecules experience this
Substance Boiling Point # of e-
N2 -196 degrees C 14
O2 -183 degrees C 16
NO - 152 degrees C 15
ICl 97 degrees C 70
Br2 59 degrees C 70
More electrons = higher the boiling point
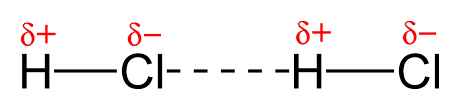
3.) Hydrogen Bonds (H-Bonds)
-This is a special type of dipole- dipole bond between H and O, F, or N
- Any molecule that: H-F, H-O or H-N
Identify the substances with H-Bonds:
1) CH4
2) CH3OH
3) H2S
4) CH3-NH2
5) HCl
6) CH2-OH-OH2
/ / /
OH OH OH
Answer: Number 2, 4, and 6
Compare the boiling points of:
- Ethanol (C2H5OH)
- Ehtane (C2H6)
- Methanol (CH3OH)
- Methane (CH4)
> London Forces are the weakest intermolecular force and hydrogen bonds are the strongest.
> More electrons, the higher the boiling point.
___________________________________________________________
IONS IN SOLUTIONS
- The formation of a solution depends on the ability of the solute to dissolve in the solvent
- Solvation is the interaction between solutes and solvents
- Ionic solids (salts) are cyrstals made up of ions
- Molecular solids are crystals made up of neutral molecules
- Dissolving ionic solutions produces ions in a process called disssociation (remember?)
Ionizaiton is the break up of a neutral molecule into charged particles
Examples:
1) FeCl3 (s) ----->Fe 3+ (aq) + 3 Cl -1 (aq)
2) Ag2O (s) -----> 2 Ag + (aq) = O 2- (aq)
Determining concentrations is relatively easy.
Cl = 3x as many moles = (0.5 M) x 3 = 1.5 M
What is the [NO3-] in a solution of 0.82 M Fe(NO3)2?
Fe(NO3)2 -----> Fe 2+ (aq) + 2 NO 3- (aq)
(0.82 M) x 2 = 1.64 M
What is the [Cr2O7 2-] and [K+] when 3.5 g of K2Cr2O7 dissolved in 40 mL of water?
K2CrO7 -----> CrO7 2- (aq) + 2K + (aq)
3.5 g x 1 mol/294.2 g = 0.0
Introduction to Solution chemistry
- Solvents are components present in larger amounts
- Solutes are componets present in smaller amounts
- A solute is soluble in a solvent if it dissolves to form a homogenous mixture
- A saturated solution contains as much solute as possible
- An unsaturated solution can dissolve more solute
- Solubility is the measure of how much solute can dissolve in a given solution (g/L, g/ml, mol/L, ppm)
- The solubility of Ba (NO3)2in water is 63 g/100 mL @ 25 degrees Celcius while the solubilty of Ba(NO3)2 in alcohol is is 1.6 g/ 100 mL @ 25 degrees Celcius
Solubility is affected by:
1. heat
2. changing the solvent
3. changing the solute
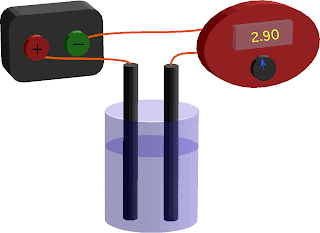
- Measuring the conductivity of a solution
APRIL 14 CLASS NOTES
- Distilled water is non-conductive
- By adding salts, we increase the conductivity
- Electrical conduction in solutions requires charged ions to be present.
- Ionic solutions dissociate (break apart) when placed in water. Molecular solutions do not usually split into ions
Ex: Dissociation of sodium chloride:
NaCl --> Na + Cl
Steps to determine conductivity:
Follow these steps to determine if the solution is conductive:
Ask...
Is it a metal?
Yes = conductive. No ask...
Is it a solid non-metal?
Yes = non-conductive. No ask...
Is it an acid or base?
Yes = conductive. If noask...
Is it ionic?
Yes = conductive. no = non-conductive.
Monday, April 5, 2010
Electronegativity [short lesson]
- Electronegativity increase from left to right and from bottom to top
http://www.chemguide.co.uk/atoms/bonding/electroneg.html
- Polaritiy is the separation of charge inside something that is neutral
Covalent bonding
- Electrons are shared between non-metals
- Drawing the Lewis Dot Diagrams:
- Total all valence electrons in all atoms
- Identify the element that can form the most bonds. This will be the central atom.
- Draw bonds between atoms as a line. This represents 2 e-
- Any e- not part of a bond are lone pairs around the atom
- Check to make sure each atom has a full octet
Double & Triple Bonds
- Some compounds form more than one bond between two elements
- Some compounds form more than one bond between two elements


Okay, YEESh.
Timmme for a much needed break from chem with some soothing tunes from the Beatles!
Atoms & Ions
- # of protons = # of electrons
- Ions have different # of protons and electrons
- Ions can be either positive (lose e) or negative (gain e)
- cation = positive ion
- anion = negative ion
Example:
C4-
Carbon gains 4 electrons and it is an anion.
CHEMICAL BONDS
- A bond is an electrostatic attraction between particles.
- Bonds occur as elements try to achieve Noble gas electron configuration
- Noble gases (usually) don't form compounds or bonds
- In Noble gases, the outermost energy level have stable octects.
- Metals lose electrons (oxidize)
- Non-metals gain electrons (reduced)
LEWIS DOT STRUCTURE
- Atoms can be represented by dot diagrams
- Dots represent electrons
- Only valeance level electrons are shown
- The atomic symbol for the atom represents the nucleus and filled inner electron levels
- One dot is used to represent outer energy level electrons
** Some helpful info:
http://www.ausetute.com.au/lewisstr.html

IONIC BONDS
- Electrons are transferred from metal to non-metal
- No dots are shown on metal
- 'Charged' species is written in brackets
Na + Cl → Na+ + Cl− → NaCl
Group projects / presentation topics
- Mendeleev's Periodic Table
- Metals
- Non-metals
- Metalloids
- Trends n Physical Properties of Elements on the Periodic Table
- Trends in chemical properties of elements on the periodic table (ion charge, chemical reactivity, ionization energy)
- Properties of Alkalis, Alkaline Earth Metals, Halogens, Noble Gases and Transition Metals
Atomic Weight [ jojojoojojoanneanneanne]
Atomic Structure / Isotopes [Joanne]
- Each element guves off a specific colour of light.
- These are known as emission spectra, unique to each element
- If electrons absorb energy they can be bumped to a higher level
- When they fall to a lower level they release that energy as light
Atomic Structure
- Atoms are made up of parts called subatomic particles
- Protons (positive), Neutrons (neutral), and Electrons (negative)
Atomic Number
= number of protons
A = atomic number
B = Ion charge
C = Symbol
E = Atomic Mass
Isotopes
- The number of protons determine the type of element
- Changing the number of neutrons changes the isotops of the elements
- All isotopes have the same chemical properties
Mass Number
Ex. Boron:
Isotope - 11
Mass - 11
Atomic # - 5
Protons - 5p
Neutrons - 6n
Ex. Nickel
Isotope - 59
Mass - 59
Atomic # - 28
Protons - 28p
Neutrons - 31n
Bohr Models [ Joanne ]
number of neutrons = atomic mass - the number of protons.
***
- Atoms are electrically neutral
- Two different models can be used to describe the electron configuartion:
1.) Energy Level Model
2.) Bohr Model
BOHR MODEL
Electrons occupy shells which are divided into orbitals
2e in the first Orbital
8e in the second orbital
8e in the third orbital
(the ones with the 8e are called Octet)
Ex. Aragon (Energy level Model)
8e- 3rd level
8e- 2nd level
2e- 1st level
40 <---atomic mass 18 Ar <---- atomic number / number of protons

 Bohr model of Argon
Bohr model of Argon____________________________________________
Ex. Chlorine (Energy Level Model)
7e- 3rd level
8e- 2nd level
2e- 1st level
35
1 Cl
2e- 1st level
19
9 F

Flourine (Bohr Model)
_____________________________
_____________________________
Hybridized orbitals
- The first of the bohr levels is the 1st orbital and it holds only 2e
the second level contains the 2s, 2px, 2py, 2pz orbitals. they combine (hybridize) to form one 2sp3 orbital
***
"OH MY GOSH,OH MY GOSH,
OHHHH MY GOSH !!!!!!"
Atomic Theory [Joanne]

____________________________________________
____________________________________________
____________________________________________
- Didn't mention the nucleus

___________________________________________
J.J. Thomson (1850s)
- Raison Bun Model
- Solid, positive spheres, with negative particles embedded in them
- First atomic theory to have positive (protons) and negative (electrons) charges
- Introduces idea of nucleus
- Didn't mention neutrons, so radioactive decay could'nt be explained
- Doesn't explain how electrons can exist outside nucleus
- Doesn't explain electrons role in chemical bonding

_________________________________________
Rutherford (1905)
- Showed that atoms have a positive, dense center with electrons outside it
- Resulted in a planetary model
- Explains why electrons spin around the nucleus
- Suggests atoms are mostly empty space
- Didn't mention neutrons
- Doesn't explain valence level electrons role in chemical bonds
__________________________________________
Bohr (1920s)
- Electrons MUST only exist in specific orbitals around nucleus
- Explains how valence elctrons are involved in bonding
- Explains the difference between ionic and covalent bonding
- Resolve the neutron (discovered in 1932)
- Explains atomic emission spectra
***
Here's a random video of an unusual singer from Filipina's got talent....
Saturday, January 23, 2010
Caloimetry and Molar Enthalpy
- Temperature change (° C)
- Amount of Water (g, kg, mL, L)
- Specific Heat Capacity (kJ/kg ° C)- the heat needed to change 1 degree C in 1 kg
ΔH = (0,400kg)(4.19kJ/kg x T)(30°C)
ΔH = 50 kJ
Molar Enthalpy
- Heat absorbed / released by one mole
Example:
When a candle (C25H25) is burnt, heat is released according to the following reaction:
1.0g (mol / 352g)
= 0.00284mol
0.00284mol × 1100kJ / 1molC25H52
= 31.2 kJ/mol
Therefore: 31.2 kJ/mol of energy are released when 1.0g of wax is burnt
HEAT AND ENTHALPY


Ø Reactions that release heat are exothermic
Ø Reactions that absorb heat are endothermic
Ø Heat is a form of energy
Ø ENTHALPY - Stored energy
Ø Enthalpy of gasoline > Enthalpy of water
Ø Enthalpy symbol is H and change in enthalpy is ΔH
ENTHALPY GRAPHS:



 EXAMPLES:
EXAMPLES:
EXOTERMIC-
2C8H18 + 25O2 -----> 16 CO2 + 18H2O + 5076 kJ
2C8H18 + 25O2 -----> 16 CO2 + 18H2O ΔH= - 5076 kJ
ENDOTHERMIC-
3.2 C + 2H2 + 52.3 kJ -----> C2H4
3.2 C + 2H2 + -----> C2H4 ΔH= - 5076 kJ
MORE Practice:
State whether each of the following are exothermic or endothermic.
a.) H + Cl ---> HCl + 432 kJ EXOTHERMIC
b.) 12CO₂ + 11H₂O ---> C1₂H₂2O11 + 12 O1₂ ΔH= 5638kJ ENDOTHERMIC
Monday, January 11, 2010
jan 8 - types of chemical reactions
A+B ----> C (Two or more substances combine)
EX: H2 + Cl2 ----> 2HCl
6) Combustion
AB---->A+B (Breaking down into simpler substances)
EX: 2Ag2O---->4Ag + O2
3) Single Replacement
A + BX---->B + AX (Compunds must have a metal and a nonmetal-replacing one atom in a compound by anohter atom)
EX: Cl2 + 2KI ----> I2 + SKCl
4) Double Replacement
AB + XY ----> AY + XB (exchange of atoms between two different compounds)
EX: 2NaCl+ H2SO4 ----> 2 HCl + Na2SO4
5) Neutralization
(Products are water and an ionic salt/ Always between acids and bases)
EX: HCl + NaOH----> NaCL + H2O
6) Combustion
There are two types: Metallic (Can also be known as a synthesis reaction/includes oxygen) and hydro-carbon (includes carbon and oxygen). Also, the productes are always CO2 and H2O
EX: C5H12 + 8 O2 ----> 5CO2 + 6 H2O
Jan 6
Balancing with C, H, & O
1.) CH4 + O2 ---> CO2 + H2O
CH4 + O2 ---> CO2 + 2H2O
2.) CHH6 + O2 ---> CO2 + H2O
2CHH6 + 7O2 ---> 4CO2 + 6H2O
3.) C8H18 + O2 ----> CO2 + H2O
2C8H18 + 25O2 ----> 16CO2 + 18H2O
ALCOHOLS:
- Octane = most important chemical used to dilate fuel gas for cars
- OH means alcohol (Ex. C2H5OH = ethane)
Examples:
1.) CH3OH + O2 ---> CO2 + H2O
2CH3OH + 3O2 ---> 2CO2 + 4H2O
2.) C2H5OH + O2 ---> CO2 +H20
C2H5OH + 3O2 ---> 2CO2 +3H20
WORDS TO BALANCED EQUATIONS:
Example 1:
Aluminum chloride is mixed with potassium carvbonate. Aluminum carbonate and potassium chloride are formed.
Write the equation and balance:
AlCl3 + K2CO3 -----> Al2(CO3)3 + KCl
2 AlCl3 + 3 K2CO3 -----> 1 Al2(CO3)3 + 6 KCl
Example 2:
Aluminum metal reacts violently with bromine to produce aluminum bromide.
Al + Br2 -----> AlBr3
2 Al + 3 Br2 -----> 2 AlBr3
Example 3:
Magnesium sulphate hepta hydratee decomposes to form water and magnesium sulphate.
MgSO4 - 7 H2O -----> MgSO4 + 7 H2O
Acids - NEED TO KNOW:
HCl - Hydrochloric Acid
HNO3 - Nitric Acid
H2SO4 - Sulphuric Acid
H3PO4 - Phosphoric Acid
CH3COOH - Acetic Acid
EXTRA Practice:
1.) NH3 + O2 ---> NO + H2O
4NH3 + 5O2 ---> 4NO + 6H2O
2.) (NH4)2C2O4 + AlCl3 ---> Al2(C2O4)2 + NH4Cl
3(NH4)2C2O4 + 2AlCl3 ---> Al2(C2O4)2 + 6NH4Cl
3.) aluminum metal reacts with bromine to form aluminum bromide
2Al + 3Br ---> 2AlBr3