Tuesday, May 18, 2010
Tuesday, May 11, 2010
May 10
- Carbon compounds can form rings
- Follow the same naming rules and add cyclo- in front of the parent chain
- When numbering, you can count clockwise or counterclockwise, but where you start (#1) should be on one of the side chains because you are using the lowest numbering system
Examples of Cyclo Alkanes:
- When a cyclic 6 carbon chain forms, it can create a resonance structure called Benzene

Monday, May 10, 2010
Organic chem
- Compounds with double bonds end in -ene
- Put a # in front of the parent chain that indicates where the double bond is
- More thn one double bond changes the parent chain slightly
- Double bond always has priority (when choosing the direction of numbering)
- Any rime more than one double bond occurs, you add adi, atri, atetra...
- The longest chain has to include the double bond

Alkynes
- For compounds with triple bonds use -yne ending
- Follow all the same alkene rules
- The longest chain has to include the triple bond

ORGANIC CHEMISTRY
- There are more carbon compounds than all ionic compounds combined
- The study of carbon compounds is called organic chemistry
- Carbon can have multiple bonds and form many different shapes
- Hydrocarbons have three types of formulas:
1) Molecular formulas
C6H14
2) Condensed Structural Formula
CH3-CH2-CH2-CH2-CH2-CH3
3) Structural Formula

Nomenclature of Hydrocarbons
- One molecular formula can have a number of different structures
- Isomers are compounds that can be drawn in more than one way
Naming Alkanes
1) Name the longest chain by using the correct suffix and adding "ane"
2) Locate any branches by number carbon atoms (use the lowest possible number system)
3) Name branches by using appropriate suffix and -yl ending (Alkyl branches)
4) If there are more than one of the same alkyl group, number each one and add the multiplier number in front of the branch name

Tuesday, April 20, 2010
April 20, 2010
Polar substances have an unequal charge distribution (asymmetrical)
H2O = polar:
LIKE DISSOLVES LIKE!
Polar solutes dissolve in polar substances and non-polar solutes dissolved in non-polar substances
___________________________________________________________
INTERMOLECULAR BONDS
- bonds between molecules
- 3 types
1.)
- Results from temporary electron dipoles
- Weakest intermolecular force
- Increases as the $ e- increases
- Occurs in any compound that has e- (ie: everything)
2.) Dipole Dipole
- Results from a permanent dipole in molecules
Polar molecules experience this force
Polarity depends how much elements want e- (electronegativity)
- Electronegativity increases to the right and up
- The strength of a dipole- dipole bond depends on the difference in electronegativity between the two atoms
- Only polar molecules experience this

Substance Boiling Point # of e-
N2 -196 degrees C 14
O2 -183 degrees C 16
NO - 152 degrees C 15
ICl 97 degrees C 70
Br2 59 degrees C 70
More electrons = higher the boiling point
3.) Hydrogen Bonds (H-Bonds)
-This is a special type of dipole- dipole bond between H and O, F, or N
- Any molecule that: H-F, H-O or H-N
Identify the substances with H-Bonds:
1) CH4
2) CH3OH
3) H2S
4) CH3-NH2
5) HCl
6) CH2-OH-OH2
/ / /
OH OH OH
Answer: Number 2, 4, and 6
Compare the boiling points of:
- Ethanol (C2H5OH)
- Ehtane (C2H6)
- Methanol (CH3OH)
- Methane (CH4)
> London Forces are the weakest intermolecular force and hydrogen bonds are the strongest.
> More electrons, the higher the boiling point.
___________________________________________________________
IONS IN SOLUTIONS
- The formation of a solution depends on the ability of the solute to dissolve in the solvent
- Solvation is the interaction between solutes and solvents
- Ionic solids (salts) are cyrstals made up of ions
- Molecular solids are crystals made up of neutral molecules
- Dissolving ionic solutions produces ions in a process called disssociation (remember?)
Ionizaiton is the break up of a neutral molecule into charged particles
Examples:
1) FeCl3 (s) ----->Fe 3+ (aq) + 3 Cl -1 (aq)
2) Ag2O (s) -----> 2 Ag + (aq) = O 2- (aq)
Determining concentrations is relatively easy.
Cl = 3x as many moles = (0.5 M) x 3 = 1.5 M
What is the [NO3-] in a solution of 0.82 M Fe(NO3)2?
Fe(NO3)2 -----> Fe 2+ (aq) + 2 NO 3- (aq)
(0.82 M) x 2 = 1.64 M
What is the [Cr2O7 2-] and [K+] when 3.5 g of K2Cr2O7 dissolved in 40 mL of water?
K2CrO7 -----> CrO7 2- (aq) + 2K + (aq)
3.5 g x 1 mol/294.2 g = 0.0
Introduction to Solution chemistry
- Solvents are components present in larger amounts
- Solutes are componets present in smaller amounts
- A solute is soluble in a solvent if it dissolves to form a homogenous mixture
- A saturated solution contains as much solute as possible
- An unsaturated solution can dissolve more solute
- Solubility is the measure of how much solute can dissolve in a given solution (g/L, g/ml, mol/L, ppm)
- The solubility of Ba (NO3)2in water is 63 g/100 mL @ 25 degrees Celcius while the solubilty of Ba(NO3)2 in alcohol is is 1.6 g/ 100 mL @ 25 degrees Celcius
Solubility is affected by:
1. heat
2. changing the solvent
3. changing the solute
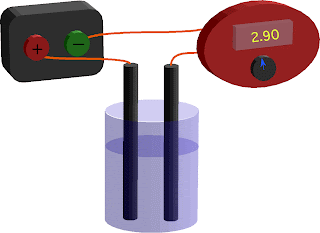
- Measuring the conductivity of a solution
APRIL 14 CLASS NOTES
- Distilled water is non-conductive
- By adding salts, we increase the conductivity
- Electrical conduction in solutions requires charged ions to be present.
- Ionic solutions dissociate (break apart) when placed in water. Molecular solutions do not usually split into ions
Ex: Dissociation of sodium chloride:
NaCl --> Na + Cl
Steps to determine conductivity:
Follow these steps to determine if the solution is conductive:
Ask...
Is it a metal?
Yes = conductive. No ask...
Is it a solid non-metal?
Yes = non-conductive. No ask...
Is it an acid or base?
Yes = conductive. If noask...
Is it ionic?
Yes = conductive. no = non-conductive.
Monday, April 5, 2010
Electronegativity [short lesson]
- Electronegativity increase from left to right and from bottom to top
http://www.chemguide.co.uk/atoms/bonding/electroneg.html
- Polaritiy is the separation of charge inside something that is neutral
Covalent bonding
- Electrons are shared between non-metals
- Drawing the Lewis Dot Diagrams:
- Total all valence electrons in all atoms
- Identify the element that can form the most bonds. This will be the central atom.
- Draw bonds between atoms as a line. This represents 2 e-
- Any e- not part of a bond are lone pairs around the atom
- Check to make sure each atom has a full octet
Double & Triple Bonds
- Some compounds form more than one bond between two elements
- Some compounds form more than one bond between two elements


Okay, YEESh.
Timmme for a much needed break from chem with some soothing tunes from the Beatles!